|
|
|
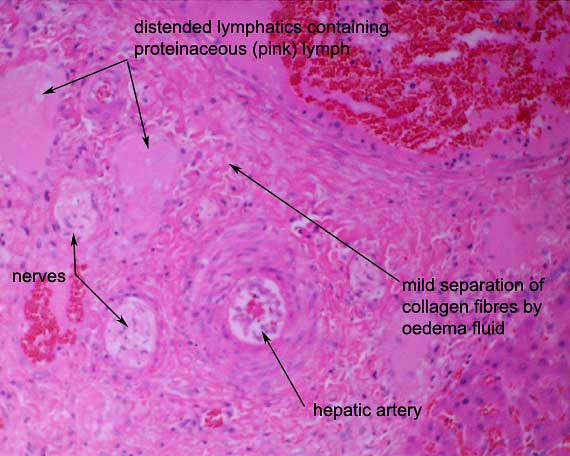
Typically in passive venous
congestion, increased hydrostatic pressure in the sinusoids drives
formation of excess hepatic lymph within the space of Disse. The excess
lymph overwhelms the lymphatic drainage pathways and pours off the surface
of the liver, leading to high protein ascites (a modified
transudate). Note the distension of lymphatics in the portal
area and the mild oedema of the connective tissue of the portal area.
Other Microscopic Findings:
Additional histological lesions included:
-
acute pulmonary oedema and congestion
-
multifocal acute skeletal myofibre degeneration and necrosis,
especially in gluteal muscles of the rump
-
acute to subacute, multifocal ventricular myocardial necrosis
-
acute necrosis of lymphocytes of splenic white pulp lymphoid
follicles, consistent with stress-induced hypercortisolaemia
The passive congestion of the lungs was consistent with
acute left-sided cardiac failure and the
passive congestion of the liver, ascites, hydrothorax and subcutaneous
ventral oedema were consistent with acute right-sided
cardiac failure.
The hepatic lesions in this foal are consistent with
acute impairment of venous outflow from the liver, with stagnation of
venous blood. Right-sided cardiac failure is the most common cause but
pericardial effusions or obstruction of efferent hepatic venous blood
flow at the level of the central veins, hepatic veins or post-hepatic
caudal vena cava can produce identical hepatic lesions.
Had the foal survived longer, there would have been
progressive atrophy or even necrosis of centrilobular hepatocytes, hydropic
or fatty degeneration of periportal and midzonal hepatocytes due to hypoxia,
and possibly fibrosis around the central veins (“cardiac
sclerosis”). These changes would have appeared grossly as
a pronounced “nutmeg liver pattern”. In chronic passive congestion
of the liver, there is usually also copious ascites and organisation of
fibrin over the liver capsule.
The combination of myocardial and skeletal myofibre
necrosis suggested several possible underlying causes, including vitamin
E/selenium deficiency (white muscle disease), exertional
rhabdomyolysis, snake envenomation
or intoxication with such agents as ionophores
or poisonous plants (e.g. avocadoes).
The hepatic selenium concentration was within the normal
equine range. Monensin was detected at a concentration of 3 ppm in large
bowel contents, permitting a diagnosis of monensin
intoxication.
Monensin and lasalocid
are polyether antibiotics produced by a Streptomyces species.
They and related, commercially produced products are used as growth promotants
in ruminants and as coccidiostats in poultry. The antibiotics are usually
added as a concentrated premix to pelleted or bulk feed. Mixing errors
or feeding ruminant or poultry feed to highly susceptible monogastric
animals may lead to intoxication. Repeat low-dosage exposure leads to
cumulative damage to skeletal and myocardial myofibres through inhibition
of membrane Na-K-ATPase pumps and consequent loss of electrolyte-mediated
calcium gating mechanisms and mitochondrial failure.
The LD in horses and other equidae is low at 2-3 mg/kg
BW and horses are the most commonly poisoned domestic species. Clinical
signs after a single toxic dose may include sudden death or a combination
of lethargy, apprehension, fidgety behaviour, stiffness, muscle weakness
(especially in the hindlimbs), muscle tremors, sweating, tachycardia,
myoglobinuria, abdominal colic and recumbency. Death is usually a consequence
of myocardial failure.
|

