Equipment / Instruments
Unit 10: Cleaning & Sterilizing
Topic 4: Sterilization Indicators
The efficacy of the sterilizing process and storage of the instruments must be monitored to ensure sterility of the equipment during the surgical procedure.
There are 3 methods of ensuring that the instruments are sterilized correctly.
1. Physical monitoring
These are attached to the autoclave and include timers, temperature and pressure gauges. Close monitoring of the physical parameters to ensure that the correct pressure and temperatures are reached are essential. Good autoclaves include an alarm system.
Regular servicing of the autoclave is also essential for maintaining the proper function of the machine.
All instrument packs should also be examined carefully prior to use to ensure that the package has not been physically damaged, or show signs of water damage.
All packs should have a date of sterilization written on them to allow us to check the date to ensure effective rotation. (Link to Unit 10.5).
2. Chemical indicators
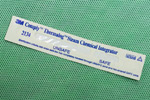
These are chemical indicators which change colour when the correct temperature is reached. They are however the least accurate as they do not indicate that the temperature has been maintained for adequate time, nor indicate that pressure levels have been reached. There are 6 classes of chemical indicators, Class 1, being the least effective (as shown in the image), with Class 6 being the most effective. Class 5 is the most commonly used in Veterinary Practice.
The most common indicators are the autoclave tapes used to externally secure the packs, or chemical strips which can be placed into the centre of the pack. Most autoclave tapes turn from a uniform colour to a tape with striped black lines.
3. Biological indicators
These are the best indicators of the sterilizers ability to function properly.
They are composed of a test vial containing non-pathogenic bacterial spores. The biological vials are then incubated for approximately 1 hour, then read off as being either safe or not safe.