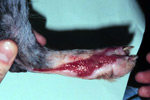
The Patient
Unit 7: Wound Healing and Management
Topic 1: The Healing Process
Our understanding of the mechanisms of wound healing has increased rapidly over the last 100 years with cellular aspects of the process having been identified, and more recently, with the development of molecular biology as a science, the molecular basis of wound healing, including the influence of various cytokines and growth factors, has become better understood.
Mammals are actually at a disadvantage to less evolved species such as amphibians in response to injury in that they have lost the power of regeneration. Mammals must depend on wound healing rather than regeneration of compound tissues.
Liver and the superficial layers of skin are the only mammalian tissues which can regenerate. Other damaged tissues are repaired by fibrous scar tissue often covered by remodeled epithelium or endothelium.
The essential processes of wound healing vary little between tissue types and occur in four overlapping and related stages:
Stage 1: Traumatic Inflammation
In general, the first two stages of wound healing are a vascular and cellular response which serve to stabilize the wound and dispose of microorganisms, foreign material and devitalized tissues.
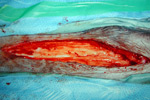
Following initial injury, bleeding will be limited and, unless severe, controlled by the activation of the blood clotting mechanisms, including the aggregation and de-granulation of platelets and the conversion of fibrinogen into interconnected strands of fibrin.
Dehydration of these strands of fibrin with other blood proteins and blood cells will result in the formation of a wound scab.
This scab provides:
- limited protection from contamination.
- maintenance of internal homeostasis.
- a surface under which epithelial cells can migrate and wound contraction may occur.
Following the initial injury haemostasis is also aided by vasoconstriction which lasts for 5-10 minutes.
Damaged tissues and de-granulating platelets will release a number of cytokines (eg PDGF and TGFß) and substances such as histamine, serotonin, kinins and prostaglandins. These have the result of producing vasodilation and the rounding of endothelial cells resulting in leaky blood vessels.
Serum containing proteins such as globulins and albumin is then allowed to enter the wound, and after 5-6 hours, the skin will develop a red, turgid and inflamed appearance.
Stage2: Destruction or Debridement
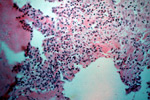
This stage begins after approximately 6 hours. Leukocytes migrate into the wound by diapedesis and start to breakdown cellular debris, bacteria and foreign material. They are attracted by factors released by platelet de-granulation.
Neutrophils migrate along the strands of fibrin and phagocytose organisms. In sterile wounds these cells degenerate releasing lysosomal enzymes from intracellular granules which attack extracellular debris to aid in its removal.
Monocytes migrate into the wound at the same time as the neutrophils. These cells become macrophages and remove most of the debris.
The macrophage also serves to attract fibroblasts into the wound by the liberation of cytokines, and are considered to have a pivotal role in the wound healing process.
Macrophages later predominate in the wound as the neutrophils are short lived.
Fluid, leukocytes, dead tissue +/- bacteria all form an inflammatory exudate resulting in the wound appearing red, swollen, hot and painful.
Stage 3: Proliferation (Repair)
The first signs of healing are seen in this stage. It involves 2 main processes:
- Fibroblast proliferation and migration
- Capillary infiltration
Fibroblast Proliferation
This only occurs after the debris has been removed, and may start 4-6 days following the injury.
Fibroblasts differentiate from mesenchymal connective tissue adjacent to the wound and migrate into the wound along a scaffolding of fibrin and fibronectin strands by cytoplasmic extensions called ruffled membranes.
They secrete various glycoproteins including collagen and protein polysaccharides to produce scar tissue. Fibroblasts require a good oxygen supply for collagen synthesis and must be within 60 m of a capillary.
Capillary Infiltration
Capillary infiltration also occurs 4-6 days following injury.
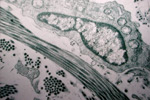
Capillary buds develop from surrounding vessels and grow into the wound as vascular loops which then interconnect and ramify throughout the wound to form a fragile network of vessels. As this develops, an oxygen gradient is created across the wound. Capillaries are stimulated to grow into areas of low oxygen tension. The endothelial cells of capillaries produce a plasminogen activator which will produce fibrinolysis concurrent with the production of collagen.
The fibroblasts, glycoproteins, protein polysaccharides and capillary loops form what is referred to as granulation tissue.
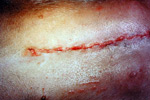
Stage 4: Maturation
After 3 weeks there is no further increase in the amount of collagen in the wound.
At this stage:
Deposition = Dissolution
Initially collagen fibres are randomly orientated. In the maturation stage of healing, the fibre bundles become orientated along the lines of stress.
Non-functional bundles undergo dissolution by collagenases produced by the endothelial cells of the capillary loops and fibroblasts in contact with the epithelial cells. New collagen is deposited by fibroblasts. More Type I collagen is produced relative to the weaker Type III collagen which initially predominated in the early granulation tissue.
The tensile strength of the wound increases significantly after about day 5 as collagen synthesis becomes apparent. There is a rapid increase in the wound strength for around 17 days, after which it slows. There may be a slow increase in strength for up to 2 years.
The increase in tensile strength is due to a number of factors:
- An increase in intra and inter-molecular cross-links between the collagen molecules
- Re-alignment of the collagen fibres
- An increase in the Type I:III ratio.
The strength of the wound however, remains 15-20% weaker than the original tissue